
Xylan is a major interlocking polysaccharide in the cell walls of seed plants, possessing a high structural diversity and cellular specificity in terms of chain polymerization, side chains, and modifications.
A group of Chinese scientists have recently reported that XYLAN O-ACETYLTRANSFERASE 6 (XOAT6) enhances xylan polymerization and folding by forming a complex with IRREGULAR XYLEM10 (IRX10), thus regulating wall strength and recalcitrance in rice.
Results of their study were published in The Plant Cell on Dec. 12.
The precise assembly of structural polysaccharides is fundamental to shaping plant architecture, as these polysaccharides form complex networks essential for mechanical support, morphogenesis, and biomass recalcitrance. Xylans incorporate most of the acetyl esters in the cell wall, which dictate xylan folding and influence its interactions with cellulose, lignin, and other wall components. To achieve this, plants have evolved sophisticated mechanisms to control xylan synthesis at multiple levels, including the formation of protein complexes.
Identifying a complex of transmembrane proteins is always a challenging task. After several years of effort and numerous attempts, ZHANG Baocai's group and ZHOU Yihua's group from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences and the collaborators successfully identified the components of XSC by performing co-fractionation mass spectrometry assay on membrane proteins extracted from rice internodes.
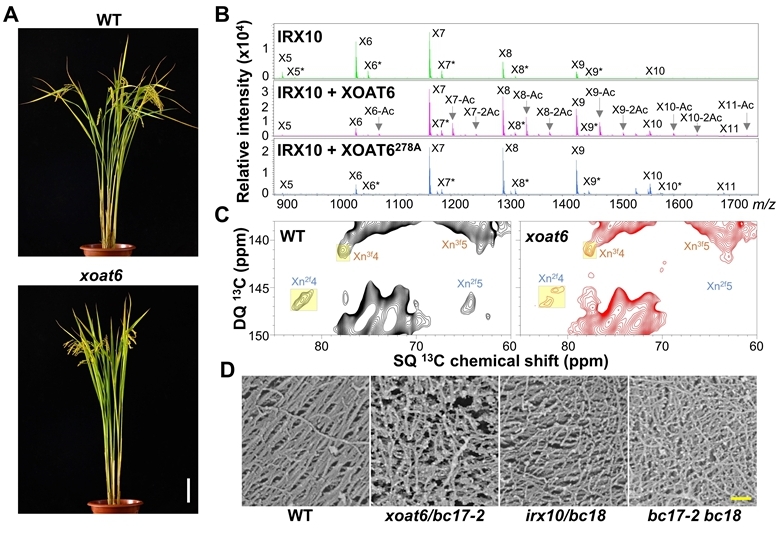
They found that XOAT6 co-fractionated with IRX10, a verified xylan synthase in rice. Further experiments, including luciferase complementation and co-immunoprecipitation assays, confirmed that IRX10 and XOAT6 interact directly.
Genetic analysis revealed that mutants deficient in IRX10 and XOAT6 exhibited similar phenotypes, including reduced acetyl ester and xylose content, which resulted in brittleness. Notably, the double mutant displayed additive effects on these phenotypes, providing genetic evidence that XOAT6 and IRX10 are crucial components of the XSC.
In vitro biochemical analyses demonstrated that XOAT6 functions as an authentic xylan acetyltransferase. Interestingly, the researchers found that recombinant XOAT6 protein can enhance the elongation of the xylan backbone mediated by IRX10, and this enhancement is not solely dependent on its acetyltransferase activity.
To obtain single-molecule level evidence, the study employed fluorescence correlation spectroscopy to visualize the xylooligomer polymerization process. Additionally, techniques such as solid-state nuclear magnetic resonance spectroscopy, field emission scanning electron microscopy, and nanoindentation analyses revealed that XOAT6 and IRX10 are vital for xylan folding and cellulose nanofibril organization, thus contributing to cell wall mechanical strength.
Moreover, mutations in XOAT6 and/or IRX10 significantly improved saccharification efficiency in assays without acid pretreatment, indicating potential applications for these findings.
This study advances our understanding of the synergistic mechanisms involved in xylan biosynthesis, particularly the coordination of backbone polymerization and acetylation modifications. The findings also offers a tool for crop trait design, biomass utilization, and the artificial synthesis of polysaccharides.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

